Research focus
Molecular and Metabolic Regulation of Bone Mass in Health and Disease

Bone is a dynamic and complex organ whose integrity is controlled by the interaction of many cell types, including osteoclasts which remove bone and osteoblasts which build it. A major area of interest is how bone cells interact with foreign invaders – in the form of tumor cells or microbes – focusing on the host as a potential therapeutic target. We use a combination of disease models, genetic mouse models, and in vitro cultures, providing a broad range of potential research projects as well as diverse technical approaches. Most of our projects are collaborative, taking advantage of the wealth of expertise in the Washington University Musculoskeletal Research Center. The research opportunities, combined with personalized mentorship, provide a positive environment in which to develop as a scientist.

Current projects
Just as the host tumor microenvironment has gained attention in the area of cancer development and spread, the field of infectious disease research has recognized that host-pathogen interactions play a significant role in the progression and therapeutic response during infection. We are now applying some of the lesions we have learned from the study of bone metastasis to the context of bacterial osteomyelitis, to elucidate the active role of bone cells in infection. We have found that S. aureus, the most common pathogen in bone infections, can replicate within cells committed to the osteoclast fate, including mature multinucleated cells. We have also developed new models of S. aureus osteomyelitis initiated by hematogenous (bloodstream) spread, using isolates from pediatric patients. Questions that we hope to address in the lab include: 1) What is the role of osteoclasts in the initiation and progression of osteomyelitis? 2) Is inflammasome activation good or bad in osteomyelitis progression? Is it different in the osteoclast and neutrophil lineages? 3) Osteomyelitis is painful. How are nerves remodeled during bone infection? Do they impact the immune response in bone?
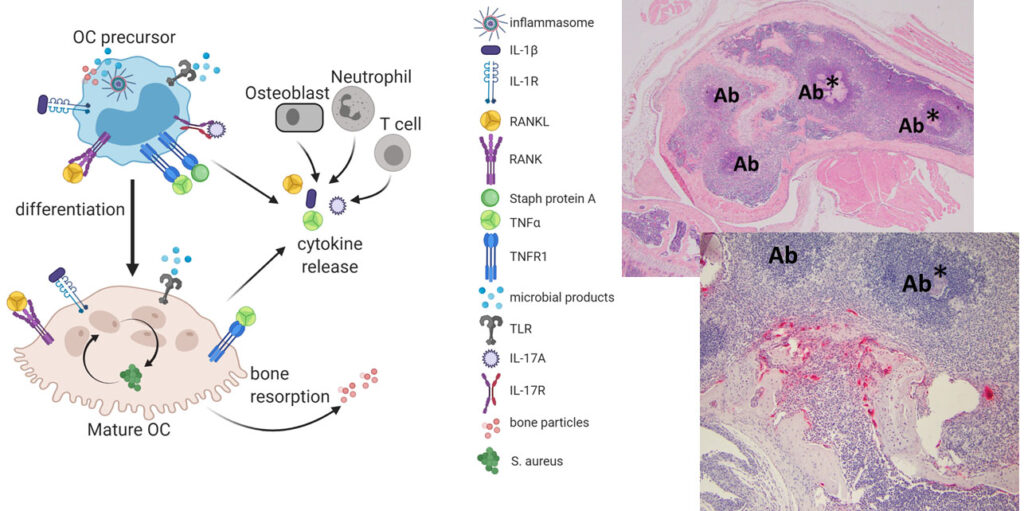
There are a wide variety of osteoclastogenic and pro-resorptive factors present in bone infection, impacting both mature osteoclasts and their precursors. In our model of hematogenous osteomyelitis, bacteria are injected into the tail vein, and within 2 weeks, abscesses (Ab) are present in the femur, some with bacterial colonies (*), and nearby osteoclasts (red cells).
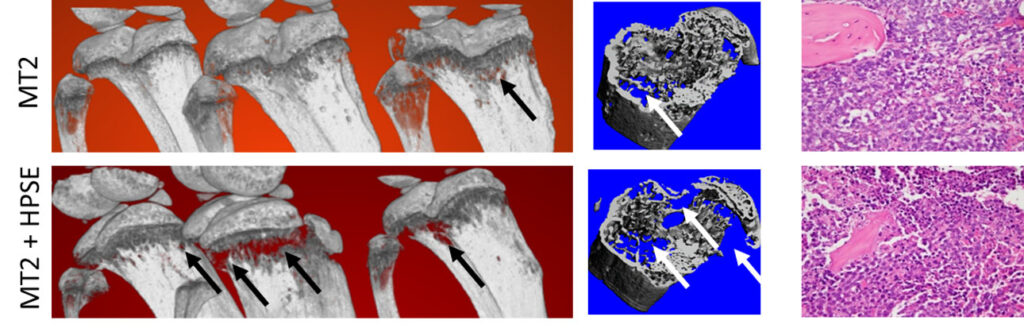
For many years, our lab has had an interest in breast cancer bone metastasis, particularly the role of the osteoclast. Although Dr. Veis maintains several collaborations in this area, recently, we have turned experimental attention towards a rare tumor that causes osteolysis, Acute T Cell Leukemia (ATL). This malignancy is caused by infection of T cells with HTLV-1, a retrovirus endemic in Asia and the Caribbean. We are using several different models of ATL – established tumor cell lines, patient-derived xenografts, HTLV-1 infected human T cells (ie. newly immortalized lines), and HTLV-1 infection of mice with humanized immune systems. These enable a variety of in vivo and in vitro systems to discover factors that mediate tumor-bone interactions. Current experiments are exploring the role of heparanase and exosomes.
Publications
Lab members
- Linda Cox, Research Assistant
- Haniya Habib, Undergraduate Student
- Luke O’Connor, PhD Candidate
- Nitin Pokhrel, PhD, Postdoc Research Associate
Join this lab
To inquire about available positions for DBBS graduate students or postdocs, please contact Dr. Veis by email at dveis@wustl.edu.
