Research focus
A Cell-Based Social Network in the Bone Microenvironment

The Civitelli Lab is interested in understanding the cellular and molecular basis of the bone remodeling process, and to devise mechanisms by which this balance can be modified. Our current research is focused on how bone cells function in a social context, via intercellular communication through gap junctions and direct cell-to-cell contact. We apply both molecular and cellular biology methods, and mouse genetics for understanding the role of connexins, cadherins and related molecules on skeletal development and maintenance. Although our research projects are basic science-oriented, they have a strong translational potential.
Current projects
We are currently working on three main lines of research, with multiple projects within each line.
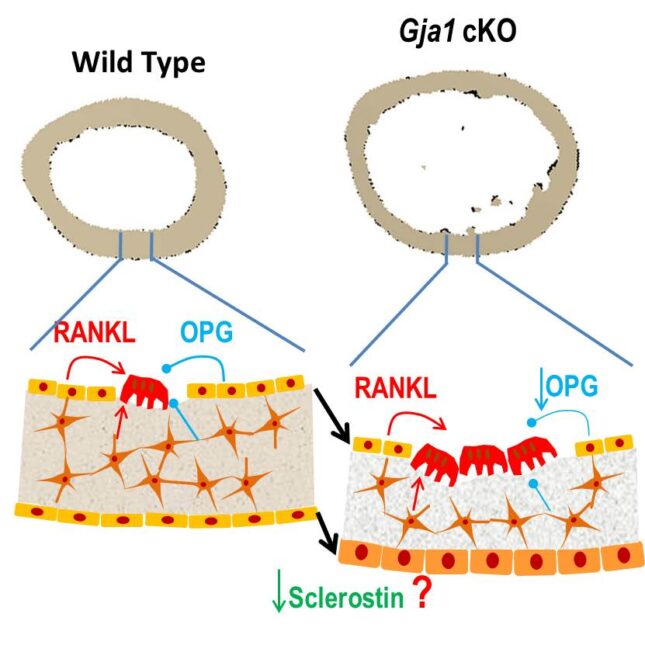
Bone forming cells, osteoblasts, express abundant gap junction proteins, primarily connexin43, but also connexin45 (Cx45). Conditional deletion of the connexin43 gene (Gja1) in bone cells alters cortical bone architecture, driven by increased endocortical bone resorption and periosteal expansion. It also renders the skeleton more sensitive to mechanical loading and unloading, causing structural changes similar to those seen in aging and disuse osteoporosis, and in the human disease oculodentodigital dysplasia, a disorder caused by Gja1 mutations.
On the other hand, ablation of the Cx45 gene (Gjc1) in osteolineage cells results in increased bone mass. In addition to forming gap junctions, Cx43 also functions as a docking platform for signaling molecules that are crucial for osteoblast function and bone formation. Using mice harboring Gja1 mutants that interfere with either the channel or signaling function of Cx43, we are currently studying the cellular and molecular mechanisms by which connexins modulate skeletal homeostasis and bone modeling during adaptive responses to hormonal and mechanical stimuli. Understanding how connexins modulate cortical bone modeling and cancellous bone mass will help devise new therapeutic approaches to counteract bone loss and microarchitectural deterioration that occur in osteoporosis, in particular disuse bone loss.
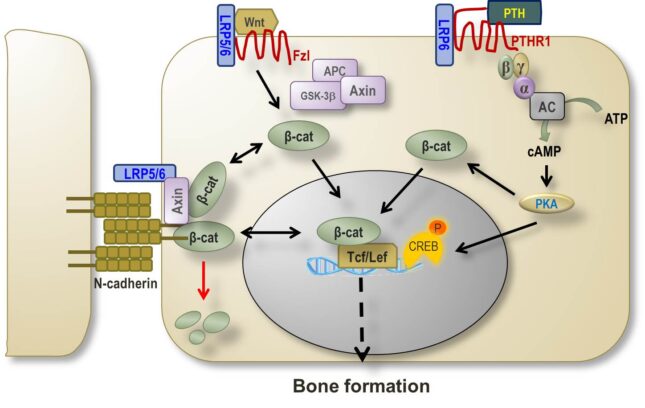
Cadherins are an integral part of adherens junctions, which mediate cell-cell adhesion. Cadherin also intersect the Wnt signaling pathway via interaction with Lrp5/6 and β-catenin. Genetic deletion of the N-cadherin (Cdh2) and cadherin-11 genes (Cdh11) causes osteopenia due to an osteoblast defect, though the two cadherins serve specific functions in bone homeostasis. Loss of Cdh2 in mesenchymal stem cells results in decreased osteoprogenitor number and growth defects. However, loss of Cdh2 in committed osteogenic cells facilitates their differentiation, and enhances the osteo-anabolic response to intermittent parathyroid hormone and Wnt activators.
We are currently working on determining how skeletal stem cells are maintained and regulated by direct contact with other cells in the bone microenvironment via cadherins, and the mechanisms by which N-cadherin interferes with Wnt signaling and with the pro-osteogenic action of Wnt signaling activators. N-cadherin may represent a target for enhancing the therapeutic window of osteo-anabolic agents.
N-cadherin is involved in migration and metastasis, and mediates adhesive interactions between tumors and stroma. While it had been shown that N-cadherin is a key factor for the establishment of the “pre-osteolytic” micrometastasis, we find that mice with ablation of the N-cadherin gene (Cdh2) in bone cells are more susceptible to develop metastases from breast or skin cancer. We are currently working on the biologic mechanism of this unexpected finding. By learning how N-cadherin modulates the tumor microenvironment we may be able to re-design strategies to target stromal support for tumor growth and metastasis.
Publications
Lab members
Consistent with the main research focus, our lab members are highly interactive and include a wide representation of geographical regions, languages and cultures that our lab community has enjoyed for many years.
- Ariella Coler-Reilly, MSTP Graduate Student
- Hillary Fujimoto, Undergrad Student
- Rhea Singal, Undergrad Student
- Toshifumi Sugatani, PhD, Staff Scientist
- Charlles Castro, MD (2000-2003)
- Dong Jin Chung, MD (2002-2004)
- Adriana Di Benedetto, PhD (2004-2006)
- Francesca Fontana, MD, PhD (2013-2018)
- Federico Furlan, MD (1999-2002)
- Susan Grimston-Engsberg, PhD (2005-2015)
- Chung-Fang Lai-Huang, PhD (2001-2003)
- Giulia Leanza, PhD
- Fernando Lecanda, PhD (1997-2000)
- Nicola Napoli, MD (2002-2005)
- Marcus Watkins, PhD (2004-2015)
- Leila Revollo, PhD (2009-2014)
- Valerie Salazar, PhD (2005-2012)
- Chan Soo Shin, MD (1998-2000)
- Joseph Stains, PhD (2001-2004)
Join this lab
To inquire about available positions for DBBS graduate students or postdocs, please contact Dr. Civitelli by email at civitellir@wustl.edu.
